Shipping calculated at checkout
Couldn't load pickup availability
Questions about this product? Ask our scientist!
Minute™ Plasma Membrane/Protein Isolation and Cell Fractionation Kit (50 Preps)
SKU:Cat #: SM-005
Manual & Protocol | MSDS
Free of detergents and EDTA, the Minute™ kit is a next-generation solution for native plasma membrane (PM) isolation and cell fractionation. It offers exceptional convenience and consistency by eliminating the variability often seen with traditional methods such as homogenization, density gradient centrifugation, and two-phase partitioning.
How it works: cells/tissues are first sensitized by buffer A before passing through the proprietary filter in a zigzag manner when high-speed centrifugal force is applied, resulting in a cell lysate containing ruptured cell membranes and intact nuclei. As a result, nuclear contamination is virtually eliminated. PM is further separated from the cell lysate (a mixture of crude membranes, intact nuclei, cytosol proteins, and organelles) by subsequent differential and density centrifugation with a regular tabletop microcentrifuge. 5 distinct cell fractions (total membrane, PM, cytosol, nucleus, and organelles) can be obtained. The procedure can be completed in less than 45 minutes.


Figure 1. A. SDS-PAGE profiles of total cell lysate (TC) and isolated plasma membrane proteins (PM) from human and rat cultured cells. Lane 1, Human lung cancer cell A549 total cell lysate; Lane 2, Isolated PM of A549; Lane 3, PM of rat REL-6TN cells; Lane 4, Total cell lysate of rat REL-6TN cells.
B. Western blotting: Proteins in A were transferred to nitrocellulose membrane and probed with anti-Na/K ATPase alpha1, a plasma membrane marker (Upstate, Clone 464.6), and anti-lamin B1, a nuclear envelope marker (ab16048, Abcam Cambridge, MA). The specific protein bands were visualized by a color metric substrate Opti-4CN (Bio-RAD).
C. Densitometry measurement of Na/K ATPase alpha1 signals in B (TC vs. PM). Total cell lysates were extracted by Minute Denaturing Total Protein Extraction Kit (SD-001, Invent Biotechnologies, Eden Prairie, MN).

Figure 2 A. SDS-PAGE profiles of isolated total membrane proteins from mouse tissues (T=Total Cell Lysate, C=Cytosol Fraction, M= Total Membrane Fraction)
B. Proteins shown in A were transferred to a nitrocellulose membrane and probed with rabbit-anti mouse pan-cadherin (ab6529, Abcam, Cambridge, MA), and anti-actin by Western blotting. The specific protein bands were visualized by a color metric substrate Opti-4CN (Bio-RAD). Signals of pan-cadherin (a 130 kDa plasma membrane marker) were significantly enhanced in total membrane protein fractions. Total cell lysates were extracted by Minute™ Denaturing Total Protein Extraction Kit (SD-001, Invent Biotechnologies, Plymouth, MN).
 Figure 3. A. SDS-PAGE profiles of total membrane protein fraction (TM) and isolated plasma membrane protein fraction from mouse tissues.
Figure 3. A. SDS-PAGE profiles of total membrane protein fraction (TM) and isolated plasma membrane protein fraction from mouse tissues.
B. Western blotting of proteins in A were transferred to nitrocellulose membrane and probed with rabbit anti-cadherin (abcam, Cambridge, MA). The specific protein bands were visualized by a color metric substrate Opti-4CN (Bio-RAD).
C. Densitometry measurement of cadherin signals in B (TM vs. PM).
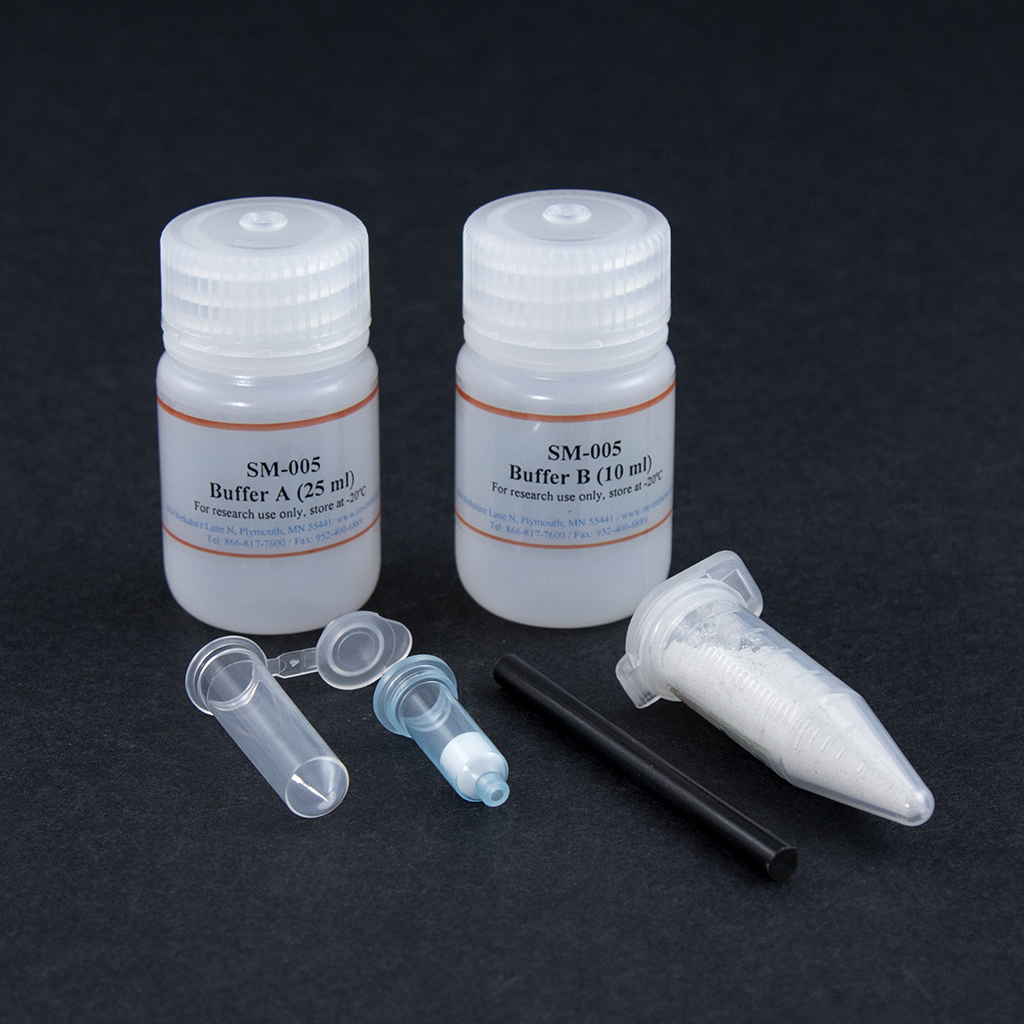
Kit includes:
|
Items |
Quantity |
|
Buffer A |
25 ml |
|
Buffer B |
10 ml |
|
Protein Extraction Filter Cartridges |
50 units |
|
Collection Tubes with Caps |
50 units |
|
Plastic Rods |
2 units |
|
Tissue Dissociation Beads |
10 grams |