Shipping calculated at checkout
Couldn't load pickup availability
Questions about this product? Ask our scientist!
Minute™ Total Protein Extraction Kit for Adipose Tissues/Cultured Adipocytes (20 Preps)
SKU:Cat #: AT-022
Manual & Protocol | MSDS
Adipose tissue, especially white adipose tissue (WAT), has been recognized as an essential endocrine and inflammation organ in addition to its energy storage function. Analyses of proteins from adipose tissues are increasingly critical for understanding many physiological/pathological conditions. However, isolation of WAT and brown adipose tissue (BAT) is technically challenging due to their high lipid and low protein contents. The water-oil emulsion present in a biological sample is notoriously difficult to separate. We have developed a novel technology to address this issue. A porous filter with unique surface property and pre-defined pore size and thickness coupling with a specially formulated detergent-free extraction buffer is employed to rapidly and effectively separate water-oil emulsion derived from adipose tissue homogenate. The extraction buffer has a lower freezing point than oil in adipose tissues, and the aqueous phase can be quickly separated from the oil phase by passing the tissue homogenate through the filter. The total proteins isolated are the unbiased representation of cellular proteins in the tissue. The extracted proteins concentration is very high (2-3 mg/ml) compared to traditional methods.
- Protein Extraction from Adipose Tissues--- A Tough Challenge
- High-Efficiency Protein Extraction from Adipose Tissues
- Novel Method for High-Quality Protein Extraction from Adipose Tissues
-

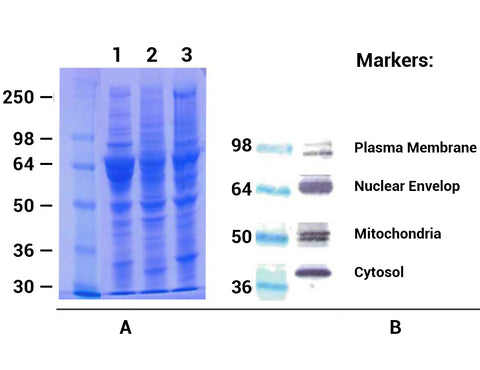
A. SDS-PAGE (10%) profiles of total protein extracted from different adipose tissues. Lane 1, porcine WAT; Lane 2, chicken BAT; Lane 3, rat WAT.
B. Western blottings of extracted proteins from rat WAT. Proteins were separated in 8-16% gradient SDS-PAGE and probed with following cellular protein marker antibodies:
Anti-Na/K ATPase alpha1, a plasma membrane marker (Upstate, clone 464.6), anti-lamin B1, a nuclear envelope marker (ab16048, abcam Cambridge, MA), anti-ubiquinol-cytochrome C reductase core protein (abcam, ab 96333) and GAPDH, a cytosolic marker (Sigma). The specific protein bands were visualized by a substrate Opti-4CN (Bio-RAD).
Kit includes:
|
Items |
Quantity |
|
Buffer A (Extraction Buffer) |
15 ml |
|
Buffer B (10 X Denaturing Buffer) |
1.5 ml |
|
Buffer C (10 X Non-Denaturing Buffer) |
1.5 ml |
|
1.5 ml Microfuge Tubes |
20 units |
|
Pestles for 1.5 ml Tubes |
2 units |
|
Filter Cartridge with Collection Tubes |
20 units |
|
Protein Extraction Powder |
2 grams |
References